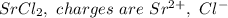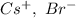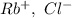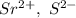Chemistry, 14.02.2020 19:38 alicemareus
The charges and sizes of the ions in an ionic compound affect the strength of the electrostatic attraction holding that compound together. Based on ion charges and relative ion sizes, rank these ionic compounds by their expected melting points from highest to lowest
a. SrCl2 ,b. CsBr ,c. RbCl ,d. SrS

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3
You know the right answer?
The charges and sizes of the ions in an ionic compound affect the strength of the electrostatic attr...
Questions




Social Studies, 07.12.2020 08:00


Mathematics, 07.12.2020 08:00


Chemistry, 07.12.2020 08:00


Mathematics, 07.12.2020 08:00



Mathematics, 07.12.2020 08:00

Mathematics, 07.12.2020 08:00

Arts, 07.12.2020 08:00

Mathematics, 07.12.2020 08:00

History, 07.12.2020 08:00


Mathematics, 07.12.2020 08:00

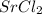 > RbCl > CsBr
> RbCl > CsBr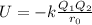
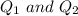 are charges and
are charges and 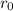 is the size.
is the size.