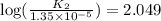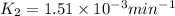Answers: 1
Another question on Chemistry


Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2


Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
The gas phase reaction 2 N2O5(g) → 4 NO2(g) + O2(g) has an activation energy of 103 kJ/mol, and the...
Questions

Mathematics, 09.06.2021 01:20




Mathematics, 09.06.2021 01:20

Social Studies, 09.06.2021 01:20


Chemistry, 09.06.2021 01:20


English, 09.06.2021 01:20



Spanish, 09.06.2021 01:20

Mathematics, 09.06.2021 01:20

Biology, 09.06.2021 01:20


Mathematics, 09.06.2021 01:20



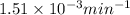
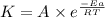
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0511/7907/6d953.png)
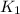 = rate constant at 266 K =
= rate constant at 266 K = 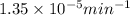
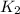 = rate constant at 296 K = ?
= rate constant at 296 K = ?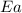 = activation energy for the reaction = 103 kJ/mol = 103000 J/mol
= activation energy for the reaction = 103 kJ/mol = 103000 J/mol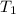 = initial temperature = 266 K
= initial temperature = 266 K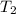 = final temperature = 296 K
= final temperature = 296 K![\log (\frac{K_2}{1.35\times 10^{-5}})=\frac{103000}{2.303\times 8.314J/mole.K}[\frac{1}{266}-\frac{1}{296}]](/tpl/images/0511/7907/d42c0.png)