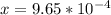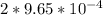Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

You know the right answer?
Hydrogen sulfide decomposes according to the following reaction, for which Kc = 9.30 × 10−8 at 700°C...
Questions

Mathematics, 20.09.2020 22:01





Mathematics, 20.09.2020 22:01



Mathematics, 20.09.2020 22:01

History, 20.09.2020 22:01

Biology, 20.09.2020 22:01

English, 20.09.2020 22:01


Mathematics, 20.09.2020 22:01


History, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01

Mathematics, 20.09.2020 22:01

English, 20.09.2020 22:01

Chemistry, 20.09.2020 22:01

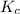 = 9.30 × 10⁻⁸
= 9.30 × 10⁻⁸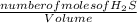
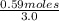
![K_c= \frac{[H_2]^2[S_2]}{[H_2S]^2}](/tpl/images/0511/9308/cf068.png)
![9.38*10^{-8}= \frac{[2x]^2[x]}{[0.1966-2x]^2}](/tpl/images/0511/9308/178bc.png)
![9.38*10^{-8}= \frac{[4x]^3}{[0.1966]^2}](/tpl/images/0511/9308/e52d1.png)
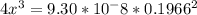
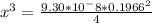
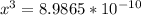
![x=\sqrt[3]{8.9865*10^{-10}}](/tpl/images/0511/9308/f6425.png)