
A solution of HNO 3 is standardized by reaction with pure sodium carbonate. 2 H + + Na 2 CO 3 ⟶ 2 Na + + H 2 O + CO 2 A volume of 26.66 ± 0.06 mL of HNO 3 solution was required for complete reaction with 0.9479 ± 0.0007 g of Na 2 CO 3 , (FM 105.988 ± 0.001 g/mol ). Find the molarity of the HNO 3 solution and its absolute uncertainty. Note: Significant figures are graded for this problem. To avoid rounding errors, do not round your answers until the very end of your calculations.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 12:10
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
A solution of HNO 3 is standardized by reaction with pure sodium carbonate. 2 H + + Na 2 CO 3 ⟶ 2 Na...
Questions

Mathematics, 18.01.2020 03:31

Mathematics, 18.01.2020 03:31


English, 18.01.2020 03:31

Social Studies, 18.01.2020 03:31

Mathematics, 18.01.2020 03:31

Physics, 18.01.2020 03:31

Mathematics, 18.01.2020 03:31


Social Studies, 18.01.2020 03:31






Chemistry, 18.01.2020 03:31

Chemistry, 18.01.2020 03:31

Biology, 18.01.2020 03:31


English, 18.01.2020 03:31

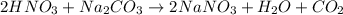
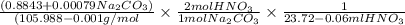
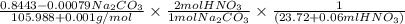
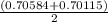 = 0.703495
= 0.703495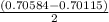
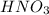 .
.

