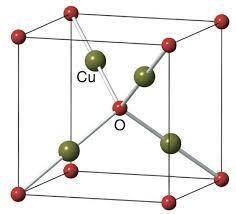
Cuprite, Cu2O, has a body-centered cubic unit cell of oxide anions with four copper cations in a tetrahedral arrangement around the body center oxide. Draw the unit cell in the empty cube. What is the coordination number and shape around the copper cations, and what is the charge on copper? Do the same analysis for the oxide anions.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1
You know the right answer?
Cuprite, Cu2O, has a body-centered cubic unit cell of oxide anions with four copper cations in a tet...
Questions



Mathematics, 02.12.2021 18:20

Mathematics, 02.12.2021 18:20

Spanish, 02.12.2021 18:20


Physics, 02.12.2021 18:20


Mathematics, 02.12.2021 18:20


History, 02.12.2021 18:20


English, 02.12.2021 18:20

Law, 02.12.2021 18:20

History, 02.12.2021 18:20


History, 02.12.2021 18:20



Mathematics, 02.12.2021 18:20