Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3


Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
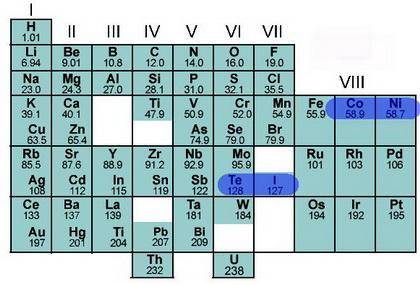
The Mendeleev and Mosley periodic charts have gaps for the as-then-undiscovered elements. Why do you...
Questions


SAT, 30.11.2021 03:50

Mathematics, 30.11.2021 03:50

Biology, 30.11.2021 03:50


Mathematics, 30.11.2021 03:50




SAT, 30.11.2021 03:50

Geography, 30.11.2021 03:50


Mathematics, 30.11.2021 03:50



Mathematics, 30.11.2021 03:50


Mathematics, 30.11.2021 03:50