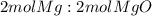Chemistry, 17.02.2020 03:02 cschuessler3
PLEASE HELP!!
Answer the following questions using the data below:
Data :Trial 1 :Trial 2
Mass of empty crucible with lid: 26.679 grams 26.698 grams
Mass of Mg metal, crucible, and lid: 26.934 grams 27.051 grams
Mass of MgO, crucible, and lid: 27.097 grams 27.274 grams
Balanced Chemical Equation for reaction: 2 MG(s) + O2(g) = 2 MGO(s)
Mass of magnesium for each trial:
Trial 1: 0.255g
Trial 2: 0.353g
Actual yield of magnesium oxide for each trial:
Trial 1: 0.418g
Trial 2: 0.576g
Question 1: Calculate the theoretical yield of MgO for each trial:
Question 2: Determine the percent yield of MgO for your experiment for each trial:
Question 3: Determine the average percent yield of MgO for the two trials:

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3


Chemistry, 23.06.2019 12:00
372 ml is the volume of aluminum, density is 2.70 g/ml what is the mass in grams
Answers: 1

You know the right answer?
PLEASE HELP!!
Answer the following questions using the data below:
Data :Tri...
Answer the following questions using the data below:
Data :Tri...
Questions

Arts, 25.11.2020 03:10

Mathematics, 25.11.2020 03:10

Mathematics, 25.11.2020 03:10

Computers and Technology, 25.11.2020 03:10

Mathematics, 25.11.2020 03:10


English, 25.11.2020 03:10

Mathematics, 25.11.2020 03:10

Mathematics, 25.11.2020 03:10








Geography, 25.11.2020 03:10


Mathematics, 25.11.2020 03:10