
Chemistry, 17.02.2020 04:05 walkereddie580
The reactant concentration in a first-order reaction was 8.10×10−2 M M after 15.0 s s and 1.80×10−3 M M after 90.0 s s . What is the rate constant for this reaction?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 23:00
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1
You know the right answer?
The reactant concentration in a first-order reaction was 8.10×10−2 M M after 15.0 s s and 1.80×10−3...
Questions



Mathematics, 14.12.2020 19:00

Mathematics, 14.12.2020 19:00


Mathematics, 14.12.2020 19:00






Chemistry, 14.12.2020 19:00

Mathematics, 14.12.2020 19:00






Mathematics, 14.12.2020 19:00

![\frac{[A]_{2} - [A]_{1} }{t_{2} - t_{1} }](/tpl/images/0512/8719/eba78.png) where [A]₁ = 8.10×10⁻² M and [A]₂ = 1.80×10⁻³ M
where [A]₁ = 8.10×10⁻² M and [A]₂ = 1.80×10⁻³ M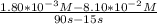 = -0.001056 M/s = -1.056×10⁻³ M/s
= -0.001056 M/s = -1.056×10⁻³ M/s

