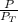A mixture of ethanol and 1−propanol behaves ideally at 36°C and is in equilibrium with its vapor. If the mole fraction of ethanol in the solution is 0.65, calculate its mole fraction in the vapor phase at this temperature. (The vapor pressures of pure ethanol and 1−propanol at 36°C are 108 and 40.0 mmHg, respectively.)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
A mixture of ethanol and 1−propanol behaves ideally at 36°C and is in equilibrium with its vapor. If...
Questions

Mathematics, 29.06.2019 19:30


Chemistry, 29.06.2019 19:30





History, 29.06.2019 19:30

History, 29.06.2019 19:30






Mathematics, 29.06.2019 19:30

History, 29.06.2019 19:30


Mathematics, 29.06.2019 19:30


History, 29.06.2019 19:30

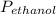 = 0.65 × 108 mmHg
= 0.65 × 108 mmHg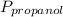 = 0.35× 40.0 mmHg
= 0.35× 40.0 mmHg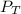 = 70.2 + 4.9 = 75.1 mmHg
= 70.2 + 4.9 = 75.1 mmHg