
Chemistry, 17.02.2020 17:16 laurenlol756
During the rGFP purification experiment, the instructor will have to make breaking buffer for the students to use. This buffer contains 150mM NaCl. Given a bottle of crystalline NaCl (M. W. = 40g/mole), describe how you would make 500ml of 150mM NaCl. Include the type and sizes of any measuring vessels that you use to make the solution.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3


Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2
You know the right answer?
During the rGFP purification experiment, the instructor will have to make breaking buffer for the st...
Questions


English, 17.03.2020 01:02

English, 17.03.2020 01:02

Mathematics, 17.03.2020 01:02


Mathematics, 17.03.2020 01:02



Mathematics, 17.03.2020 01:03


Mathematics, 17.03.2020 01:03

Mathematics, 17.03.2020 01:03

Social Studies, 17.03.2020 01:03

Computers and Technology, 17.03.2020 01:08

Mathematics, 17.03.2020 01:08

History, 17.03.2020 01:08


Health, 17.03.2020 01:08


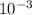 m
m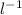 × 500×
× 500×


