
Chemistry, 17.02.2020 17:26 sedilei1515
Two equilibrium reactions of nitrogen with oxygen, with their corresponding equilibrium constants (Kc) at a certain temperature, are given below. reaction (1): N2(g) + O2(g) 2 NO(g); Kc = 1.54e-31 reaction (2): N2(g) + 1/2 O2(g) N2O(g); Kc = 2.61e-24 Using this set of data, determine the equilibrium constant for the following reaction, at the same temperature. reaction (3): N2O(g) + 1/2 O2(g) 2 NO(g)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 02:00
What can be done to make a solid solute dissolve faster in a liquid solvent?
Answers: 1
You know the right answer?
Two equilibrium reactions of nitrogen with oxygen, with their corresponding equilibrium constants (K...
Questions

Computers and Technology, 10.07.2019 22:00

Business, 10.07.2019 22:00


Mathematics, 10.07.2019 22:00



History, 10.07.2019 22:00



Mathematics, 10.07.2019 22:00


Spanish, 10.07.2019 22:00

Mathematics, 10.07.2019 22:00

Mathematics, 10.07.2019 22:00




Mathematics, 10.07.2019 22:00

Mathematics, 10.07.2019 22:00

German, 10.07.2019 22:00

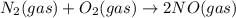 ; Kc = 1.54e - 31
; Kc = 1.54e - 31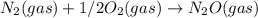 ; Kc = 2.16e - 24
; Kc = 2.16e - 24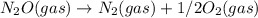 Kc = 1/2.16e - 24
Kc = 1/2.16e - 24 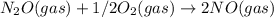 Kc = 1.54e-31 × 1/2.61e - 24
Kc = 1.54e-31 × 1/2.61e - 24

