Chemistry, 17.02.2020 17:27 hiplikedyani
Calculate the amount of heat released in the combustion of 10.5 grams of Al with 3 grams of O2 to form Al2O3(s) at 25°C and 1 atm. ΔHfAl2O3(s) = −1676 kJ/mol HINT: What does ΔHfAl2O3(s) mean?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

You know the right answer?
Calculate the amount of heat released in the combustion of 10.5 grams of Al with 3 grams of O2 to fo...
Questions

English, 21.07.2019 10:20

Business, 21.07.2019 10:20

Business, 21.07.2019 10:20

Social Studies, 21.07.2019 10:20

Arts, 21.07.2019 10:20


History, 21.07.2019 10:20

Physics, 21.07.2019 10:20


Mathematics, 21.07.2019 10:20






History, 21.07.2019 10:20



Biology, 21.07.2019 10:20

Spanish, 21.07.2019 10:20

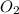 .
.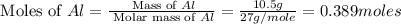
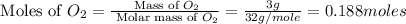
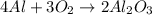
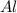
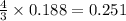 moles of
moles of 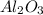
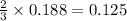 moles of
moles of