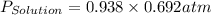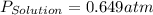Chemistry, 17.02.2020 17:57 Alienhead6187
12. The vapor pressure of water at 90°C is 0.692 atm. What is the vapor pressure (in atm) of a solution made by dissolving 3.68 mole(s) of CsF(s) in 1.00 kg of water? Assume that Raoult's law applies.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
The most efficient way to establish the best possible economizer position is to measure
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1
You know the right answer?
12. The vapor pressure of water at 90°C is 0.692 atm. What is the vapor pressure (in atm) of a solut...
Questions

Arts, 03.12.2020 09:20

Arts, 03.12.2020 09:20




Mathematics, 03.12.2020 09:20


Computers and Technology, 03.12.2020 09:20

Computers and Technology, 03.12.2020 09:20




Mathematics, 03.12.2020 09:20


Spanish, 03.12.2020 09:20

Chemistry, 03.12.2020 09:20

English, 03.12.2020 09:20

Mathematics, 03.12.2020 09:20

Mathematics, 03.12.2020 09:20

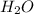 = 1.00 kg = 1000 g
= 1.00 kg = 1000 g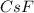 = 3.68 mole
= 3.68 mole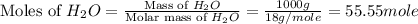
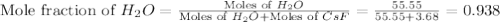
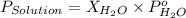
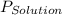 = vapor pressure of solution
= vapor pressure of solution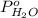 = vapor pressure of water = 0.692 atm
= vapor pressure of water = 0.692 atm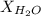 = mole fraction of water = 0.938
= mole fraction of water = 0.938