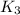The following two-step process has equilibrium constants K1 and K2. Step 1: H2(g) + ICl(g) → HI(g) + HCl(g) K1 Step 2: HI(g) + ICl(g) → HCl(g) + I2(g) K2 Overall : H2(g) + 2ICl(g) → 2HCl(g) + I2(g) K3 What is the expression for the equilibrium constants for the overall reaction, K3?

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of sulfur dioxide are in 2.26 × 10^33 sulfur dioxide molecules?
Answers: 3

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
The following two-step process has equilibrium constants K1 and K2. Step 1: H2(g) + ICl(g) → HI(g) +...
Questions

Computers and Technology, 30.07.2019 09:30

Mathematics, 30.07.2019 09:30

Geography, 30.07.2019 09:30


Biology, 30.07.2019 09:30



English, 30.07.2019 09:30

Health, 30.07.2019 09:30



Chemistry, 30.07.2019 09:30

Spanish, 30.07.2019 09:30

History, 30.07.2019 09:30

Biology, 30.07.2019 09:30



World Languages, 30.07.2019 09:30


Chemistry, 30.07.2019 09:30

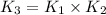
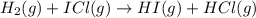 ;
; 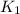
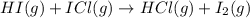 ;
; 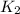
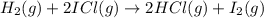 ;
;