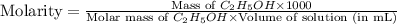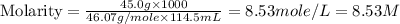Chemistry, 17.02.2020 19:39 nikki987654
The density of a 45.0 mass % solution of ethanol (C2H5OH) in water is 0.873 g/mL. What is the molarity of the solution?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2
You know the right answer?
The density of a 45.0 mass % solution of ethanol (C2H5OH) in water is 0.873 g/mL. What is the molari...
Questions


Biology, 19.03.2022 21:00



Advanced Placement (AP), 19.03.2022 21:00


Mathematics, 19.03.2022 21:30





Social Studies, 19.03.2022 21:50


SAT, 19.03.2022 21:50

History, 19.03.2022 22:00

Social Studies, 19.03.2022 22:00

Computers and Technology, 19.03.2022 22:10



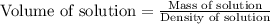
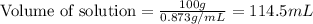
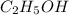 = 45.0 g
= 45.0 g