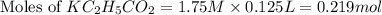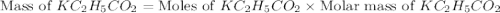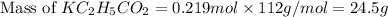Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3
You know the right answer?
A chemistry graduate student is given 125. mL of a 1.30 M propanoic acid (HC2H, Co2) solution. Propa...
Questions

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Chemistry, 22.10.2020 01:01

Engineering, 22.10.2020 01:01


History, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Business, 22.10.2020 01:01


Social Studies, 22.10.2020 01:01

History, 22.10.2020 01:01


Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01



Mathematics, 22.10.2020 01:01

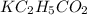 is, 24.5 grams
is, 24.5 grams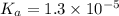
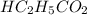 = 1.30 M
= 1.30 M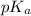 .
.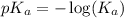
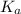 in this expression, we get:
in this expression, we get: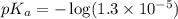
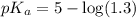
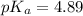
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0513/2213/e961a.png)
![pH=pK_a+\log \frac{[KC_2H_5CO_2]}{[HC_2H_5CO_2]}](/tpl/images/0513/2213/845a9.png)
![5.02=4.89+\log (\frac{[KC_2H_5CO_2]}{1.30})](/tpl/images/0513/2213/18fd8.png)
![[KC_2H_5CO_2]=1.75M](/tpl/images/0513/2213/46461.png)