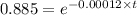Chemistry, 17.02.2020 22:21 timothycarter342
Carbon-14 has a half-life of 5720 years and this is a first-order reaction. If a piece of wood has converted 11.5% of the carbon-14, then how old is it? 4290 years 2375 years 17160 years 4750 years 1008 years

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the cellular process that releases the energy stored in food molecules
Answers: 3


Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

You know the right answer?
Carbon-14 has a half-life of 5720 years and this is a first-order reaction. If a piece of wood has c...
Questions











History, 22.01.2020 20:31


Biology, 22.01.2020 20:31


Computers and Technology, 22.01.2020 20:31

Business, 22.01.2020 20:31




Computers and Technology, 22.01.2020 20:31



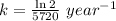
![[A_t]=[A_0]e^{-kt}](/tpl/images/0513/4232/1ef89.png)
![[A_t]](/tpl/images/0513/4232/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0513/4232/9a686.png) is the initial concentration
is the initial concentration
![\frac {[A_t]}{[A_0]}](/tpl/images/0513/4232/0d33c.png) = 1 - 0.115 = 0.885
= 1 - 0.115 = 0.885![\frac {[A_t]}{[A_0]}=e^{-k\times t}](/tpl/images/0513/4232/16cf4.png)