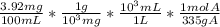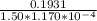Chemistry, 17.02.2020 23:29 alishabhappy1
A solution containing 3.92 mg/100 mL of A (335 g/mol) has a transmittance of 64.1% in a 1.50-cm cell at 425 nm. Calculate the molar absorptivity of A at this wavelength.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2


Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1
You know the right answer?
A solution containing 3.92 mg/100 mL of A (335 g/mol) has a transmittance of 64.1% in a 1.50-cm cell...
Questions



Mathematics, 21.06.2019 16:00

Geography, 21.06.2019 16:00

Geography, 21.06.2019 16:00

English, 21.06.2019 16:00



Mathematics, 21.06.2019 16:00


History, 21.06.2019 16:00



History, 21.06.2019 16:00




Mathematics, 21.06.2019 16:00

Mathematics, 21.06.2019 16:00