
Chemistry, 18.02.2020 01:06 morronefamily1
Be sure to answer all parts. Rank the following groups in order of decreasing priority: A. −COOH B. −H C. −NH2 D. −OH

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2
You know the right answer?
Be sure to answer all parts. Rank the following groups in order of decreasing priority: A. −COOH B....
Questions

Mathematics, 27.06.2019 07:10

English, 27.06.2019 07:10

Health, 27.06.2019 07:10


Mathematics, 27.06.2019 07:10

English, 27.06.2019 07:10



Mathematics, 27.06.2019 07:10



Mathematics, 27.06.2019 07:10



English, 27.06.2019 07:10

Mathematics, 27.06.2019 07:10

Mathematics, 27.06.2019 07:10


Mathematics, 27.06.2019 07:10

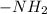 > -COOH > -H
> -COOH > -H

