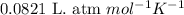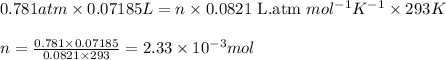Chemistry, 18.02.2020 01:59 MariaGuerra
You analyze a sample of unknown metal as you would in this experiment. You measure the volume of H2(g) generated to be 71.85 mL and the water temperature to be 20.0°C. You calculate PH2 to be 0.781 atm. Use the ideal gas law to calculate the number of moles of hydrogen gas generated.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1


Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
You analyze a sample of unknown metal as you would in this experiment. You measure the volume of H2(...
Questions



Mathematics, 20.11.2020 01:00

History, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

Spanish, 20.11.2020 01:00

English, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

Health, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

Physics, 20.11.2020 01:00



Mathematics, 20.11.2020 01:00

Physics, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00


Law, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

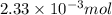
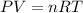
![20^oC=[20+273]K=293K](/tpl/images/0513/7044/3b5d4.png)