

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:10
Seawater contains approximately 3.5%nacl by mass and has a density of 1.02 g/ml. what volume of seawater contains 7.5 g of sodium?
Answers: 2

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2
You know the right answer?
Consider the following multistep reaction:
C+D⇌CD(fast)
CD+D→CD2(slow)
CD...
C+D⇌CD(fast)
CD+D→CD2(slow)
CD...
Questions



English, 26.08.2021 05:40


Mathematics, 26.08.2021 05:40

Mathematics, 26.08.2021 05:40

History, 26.08.2021 05:40



English, 26.08.2021 05:40



History, 26.08.2021 05:40

Mathematics, 26.08.2021 05:40


Mathematics, 26.08.2021 05:40

Mathematics, 26.08.2021 05:40



Mathematics, 26.08.2021 05:40

![\text{Rate}=k'[C][D]^2](/tpl/images/0513/7830/4eb88.png)
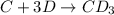
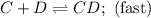
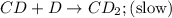
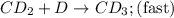
![\text{Rate}=k[CD][D]](/tpl/images/0513/7830/52b27.png) ......(1)
......(1)![K=\frac{[CD]}{[C][D]}](/tpl/images/0513/7830/c2f5d.png)
![[CD]=K[C][D]](/tpl/images/0513/7830/b2574.png)
![\text{Rate}=k.K[C][D]^2\\\\\text{Rate}=k'[C][D]^2](/tpl/images/0513/7830/6c083.png)


