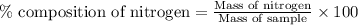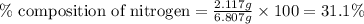Chemistry, 18.02.2020 03:15 jazzycintron14
A 6.807g sample of an unknown compound, composed only of carbon, hydrogen, and nitrogen, produced 13.3g of CO2 and 9.52g of H2O in a combustion analysis. What is the mass percent composition of nitrogen of the unknown compound?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
The crust of earth may a- continets and ocean floors. b-continents only. c-layers of sedimentary rocks and continents. d-all of the above
Answers: 2

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 12:00
the mississippians were considered to be horticulturalists, which means they were
Answers: 1
You know the right answer?
A 6.807g sample of an unknown compound, composed only of carbon, hydrogen, and nitrogen, produced 13...
Questions


Mathematics, 11.03.2020 03:11

Physics, 11.03.2020 03:11










Mathematics, 11.03.2020 03:11






Mathematics, 11.03.2020 03:11

Mathematics, 11.03.2020 03:11

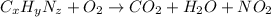
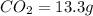
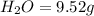
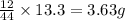 of carbon will be contained.
of carbon will be contained.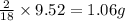 of hydrogen will be contained.
of hydrogen will be contained.