
Chemistry, 18.02.2020 03:29 onewaydemon
The iodide ion reacts with hypochlorite ion (the active ingredient in chlorine bleaches) in the following way:
OCl- + I- → OI-1 +Cl-.
This rapid reaction gives the following rate data:
[OCl-](M) [I]- (M) Rate (M/s)
1.5×10^3 1.5×10^3 1.36×10^4
3.0×10^3 1.5×10^3 2.72×10^4
1.5×10^3 3.0×10^3 2.72×10^4
Write the rate law for this reaction.
Calculate the rate constant with proper units.
Calculate the rate when [OCl-]= 1.8×10^3 M and [I-]= 6.0×10^4 M .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1


Chemistry, 23.06.2019 17:30
With carbon dioxide what phase change take place when the temperature decreases from -40c to -80c at 2 atm
Answers: 2
You know the right answer?
The iodide ion reacts with hypochlorite ion (the active ingredient in chlorine bleaches) in the foll...
Questions




Chemistry, 21.04.2021 07:00

Spanish, 21.04.2021 07:00



History, 21.04.2021 07:00

Mathematics, 21.04.2021 07:00

Mathematics, 21.04.2021 07:00

Mathematics, 21.04.2021 07:00


Social Studies, 21.04.2021 07:00

Chemistry, 21.04.2021 07:00

Social Studies, 21.04.2021 07:00


Social Studies, 21.04.2021 07:00

Biology, 21.04.2021 07:00


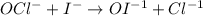
![K \times [OCl^{-}] \times [l^{-}]](/tpl/images/0513/8261/88013.png)
![K [OCl^{-}][l^{-}]](/tpl/images/0513/8261/94fd7.png)
![[OCl^{-}] = [l^{-}]](/tpl/images/0513/8261/5e8f1.png) )
)
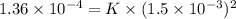
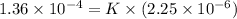
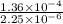
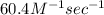
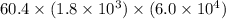
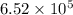
![[OCl^{-}] = 1.8 \times 10^{3}](/tpl/images/0513/8261/c42db.png) M and
M and ![[I^{-}]= 6.0 \times 10^{4}](/tpl/images/0513/8261/40481.png) M is
M is 


