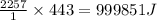Chemistry, 18.02.2020 06:27 rickyortega72701
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature. On average, an athlete loses approximately 443 g of sweat during an hour of exercise. How much energy is needed to evaporate the sweat that is produced? The heat of vaporization for water is 2257 J/g.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2
You know the right answer?
As an athlete exercises, sweat is produced and evaporated to help maintain a proper body temperature...
Questions


Mathematics, 25.08.2019 01:00

History, 25.08.2019 01:00



Mathematics, 25.08.2019 01:00

Social Studies, 25.08.2019 01:00

Chemistry, 25.08.2019 01:00


Mathematics, 25.08.2019 01:00

Biology, 25.08.2019 01:00

Business, 25.08.2019 01:00

Social Studies, 25.08.2019 01:00



Mathematics, 25.08.2019 01:00

Geography, 25.08.2019 01:00


Chemistry, 25.08.2019 01:00

Computers and Technology, 25.08.2019 01:00