
The rate law for the reaction
2NO(g) + Cl2(g) → 2NOCl(g)
is given by R = k[NO][Cl2]
If the following is the mechanism for the reaction, NO(g) + Cl2(g) → NOCl2(g) NOCl2(g) + NO(g) → 2NOCl(g)
which of the following statements accurately describes this reaction? Check all that apply.
-2nd order reaction
-The first step is the slow step.
-Doubling [NO] would quadruple the rate.
-Cutting [Cl2] in half would decrease the rate by a factor of two.
-The molecularity of the first step is 1.
-Both steps are termolecular.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1


Chemistry, 22.06.2019 20:10
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
The rate law for the reaction
2NO(g) + Cl2(g) → 2NOCl(g)
is given by R = k...
2NO(g) + Cl2(g) → 2NOCl(g)
is given by R = k...
Questions




Business, 17.05.2021 19:10

Mathematics, 17.05.2021 19:10




English, 17.05.2021 19:10

Chemistry, 17.05.2021 19:10






Social Studies, 17.05.2021 19:10


Computers and Technology, 17.05.2021 19:10

History, 17.05.2021 19:10

Mathematics, 17.05.2021 19:10

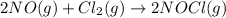
![k[NO][Cl_{2}]](/tpl/images/0514/1761/8cd0b.png)
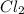 . Since, power of the concentrations of each of these is equal to 1. Therefore, order of the reaction is equal to 1 + 1 = 2.
. Since, power of the concentrations of each of these is equal to 1. Therefore, order of the reaction is equal to 1 + 1 = 2.

