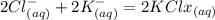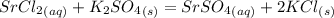When an aqueous solution of strontium chloride is added to an aqueous solution of potassium sulfate, a precipitation reaction occurs. Write the balanced net ionic equation of the reaction. Include charges on the ions, where applicable. Include coefficients only when they are different than ?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3


Chemistry, 23.06.2019 06:00
Jenny wants to test the electrical conductivity of two substances dissolved in water. she is preparing the containers for the experiment. which factor is most important for her to control?
Answers: 1
You know the right answer?
When an aqueous solution of strontium chloride is added to an aqueous solution of potassium sulfate,...
Questions

Mathematics, 02.08.2019 09:10




History, 02.08.2019 09:10

Biology, 02.08.2019 09:10


Mathematics, 02.08.2019 09:10

Mathematics, 02.08.2019 09:10

Business, 02.08.2019 09:10

Biology, 02.08.2019 09:10

Biology, 02.08.2019 09:10

Business, 02.08.2019 09:10

Social Studies, 02.08.2019 09:10


Biology, 02.08.2019 09:10

Biology, 02.08.2019 09:10

History, 02.08.2019 09:10


History, 02.08.2019 09:10