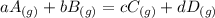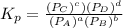Chemistry, 18.02.2020 19:34 iliketurtures
The equilibrium pressures below were observed at a certain temperature for the following reaction.
PNH₃ = 3.1 ✕ 10⁻² atm
PN₂ = 8.5 ✕ 10⁻¹ atm
PH₂ = 3.1 ✕ 10⁻³ atm
Calculate the value for the equilibrium constant at this temperature.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Three alkali metals in group 1 are a. calcium, strontium, barium b. boron, aluminum, gallium c. sodium, potassium, rubidium d. fluorine, iodine, chlorine
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
The equilibrium pressures below were observed at a certain temperature for the following reaction. <...
Questions




Mathematics, 09.10.2021 08:10

Chemistry, 09.10.2021 08:10

History, 09.10.2021 08:10


English, 09.10.2021 08:10


Physics, 09.10.2021 08:10


Chemistry, 09.10.2021 08:10

Chemistry, 09.10.2021 08:10





Biology, 09.10.2021 08:10

Mathematics, 09.10.2021 08:10