
Chemistry, 18.02.2020 19:53 hannahking1869
The 116-g sample was heated to 94.5°C and placed into a calorimeter containing 72 g of water at 20.0°C. The heat capacity of the calorimeter was 14.7 J/K. The final temperature in the calorimeter was 25.6°C. What is the specific heat capacity (in J/g°C) of the mineral?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:50
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

You know the right answer?
The 116-g sample was heated to 94.5°C and placed into a calorimeter containing 72 g of water at 20.0...
Questions

Mathematics, 20.10.2019 14:10


Biology, 20.10.2019 14:10

Chemistry, 20.10.2019 14:10

History, 20.10.2019 14:10


Mathematics, 20.10.2019 14:10

English, 20.10.2019 14:10


Mathematics, 20.10.2019 14:10

Spanish, 20.10.2019 14:10


Mathematics, 20.10.2019 14:10

Mathematics, 20.10.2019 14:10


Chemistry, 20.10.2019 14:10

Mathematics, 20.10.2019 14:10

Social Studies, 20.10.2019 14:10

Physics, 20.10.2019 14:10

English, 20.10.2019 14:10

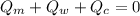
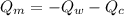 ----------------equation (1)
----------------equation (1)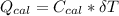 ---------------- equation (2)
---------------- equation (2)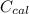 = heat capacity of the calorimeter
= heat capacity of the calorimeter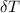 = change in temperature
= change in temperature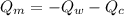
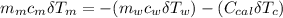
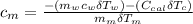 ---------------- equation (4)
---------------- equation (4)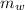 ) = 72g
) = 72g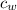 ) = 4.18 J/g.k
) = 4.18 J/g.k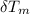 ) = 25.6 °C - 94.5 °C
) = 25.6 °C - 94.5 °C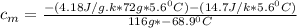
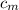 = 0.221172 J/g°C
= 0.221172 J/g°C

