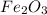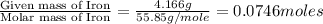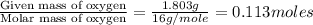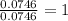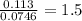Chemistry, 18.02.2020 23:31 navleen4owjrk1
In a reaction involving iron, Fe, and oxygen, O. it was determined that 4.166 grams of iron reacted with 1.803 grams of oxygen. From this information, determine the empirical formula of the compound that resulted. a. FEO2b. FeO3c. Fe2Od. Fe2O3

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:30
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 23.06.2019 02:00
To calculate the molarity of a solution, you need to know the moles of solute and the
Answers: 2

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1
You know the right answer?
In a reaction involving iron, Fe, and oxygen, O. it was determined that 4.166 grams of iron reacted...
Questions



















Social Studies, 18.12.2019 03:31