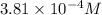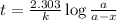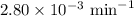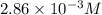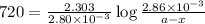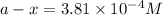G The gas phase decomposition of sulfuryl chloride at 600 K SO2Cl2(g) SO2(g) + Cl2(g) is first order in SO2Cl2 with a rate constant of 2.80×10-3 min-1. If the initial concentration of SO2Cl2 is 2.86×10-3 M, the concentration of SO2Cl2 will be M after 720 min have passed.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no2]2 ? second order 3/2 order third order zero order none of the listed answers are correct
Answers: 3

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 20:00
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
G The gas phase decomposition of sulfuryl chloride at 600 K SO2Cl2(g) SO2(g) + Cl2(g) is first order...
Questions



Mathematics, 31.03.2020 03:07



Mathematics, 31.03.2020 03:07

Mathematics, 31.03.2020 03:07

English, 31.03.2020 03:07






Mathematics, 31.03.2020 03:17

English, 31.03.2020 03:17





Biology, 31.03.2020 03:17