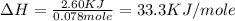Chemistry, 19.02.2020 00:21 carlyfaith3375
When 60 mL of 1.30 mol/L AgNO3(aq) and 60 mL of 1.30 mol/L HCl(aq) are mixed in a simple calorimeter, the temperature rises by 5.18°C. The molar enthalpy of reaction of HCl(aq) is ab. C kJ/mol.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1

Chemistry, 23.06.2019 09:50
When scientists are ready to publish the results of their experimentation, why is it important for them to include a description of the procedures they used?
Answers: 1
You know the right answer?
When 60 mL of 1.30 mol/L AgNO3(aq) and 60 mL of 1.30 mol/L HCl(aq) are mixed in a simple calorimeter...
Questions

Engineering, 19.10.2020 05:01

Mathematics, 19.10.2020 05:01



Mathematics, 19.10.2020 05:01

Biology, 19.10.2020 05:01

History, 19.10.2020 05:01




History, 19.10.2020 05:01

Mathematics, 19.10.2020 05:01




Mathematics, 19.10.2020 05:01

English, 19.10.2020 05:01

Mathematics, 19.10.2020 05:01

Health, 19.10.2020 05:01

Mathematics, 19.10.2020 05:01

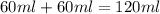
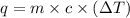
 = specific heat of water =
= specific heat of water = 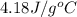
 = change in temperature =
= change in temperature = 
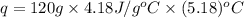
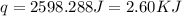
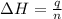
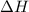 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?