
Chemistry, 19.02.2020 00:56 rouchedavisin4
For a reaction with ΔH° = −9 kJ/mol, decide if the following statement is true. If it is false, choose the statement that makes it true. Select the single best answer. The reaction is exothermic. (Assume the reaction has a small entropy term compared to the enthalpy term) a. The statement is false. b. The reaction does not occur. c. The statement is true. d. The statement is false. e. The reaction is endergonic. f. The statement is false. g. The reaction is endothermic.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2

Chemistry, 23.06.2019 02:00
Anitrogen atom and an oxygen atom combine chemically to form nitric oxide. what is nitric oxide?
Answers: 1
You know the right answer?
For a reaction with ΔH° = −9 kJ/mol, decide if the following statement is true. If it is false, choo...
Questions


Mathematics, 18.07.2019 09:00



Mathematics, 18.07.2019 09:00


Mathematics, 18.07.2019 09:00


Mathematics, 18.07.2019 09:00

English, 18.07.2019 09:00

Mathematics, 18.07.2019 09:00


Mathematics, 18.07.2019 09:00

Mathematics, 18.07.2019 09:00





History, 18.07.2019 09:00

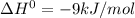 , the heat is released and is exothermic.Thus the statement that the reaction is exothermic is true.
, the heat is released and is exothermic.Thus the statement that the reaction is exothermic is true.

