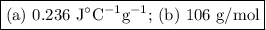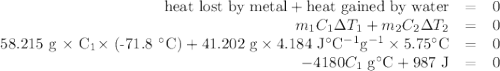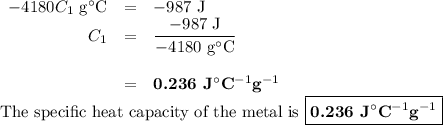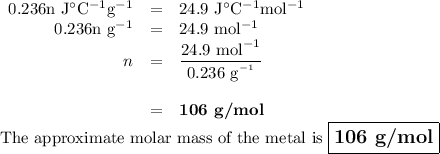Chemistry, 19.02.2020 01:04 larenhemmings
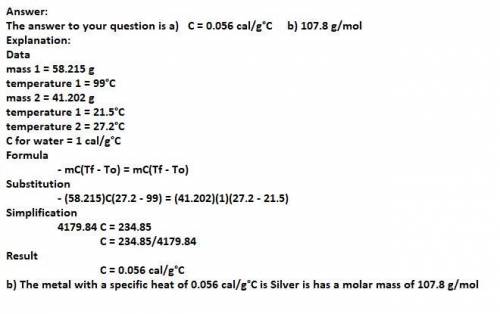
A 58.215 g sample of a pure metal is brought to 99.0c and added o 41.202 g of water at 21.5c in a calorimeter. if the metal and water arrive at a final, equal temerature of 27.2c find a) the specific heat of the metal, and b) the approximate molar mass of yhe metal

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1
You know the right answer?
A 58.215 g sample of a pure metal is brought to 99.0c and added o 41.202 g of water at 21.5c in a ca...
Questions




Mathematics, 18.12.2019 00:31



Mathematics, 18.12.2019 00:31

English, 18.12.2019 00:31