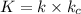Chemistry, 19.02.2020 01:25 NetherisIsTheQueen
The following mechanism has been proposed for the gas phase reaction of nitrogen monoxide with bromine.
step 1 fast: NO Br2 NOBr2
step 2 slow: NOBr2 NO 2 NOBr
(1) What is the equation for the overall reaction
(2) Enter the formula of any species that acts as a reaction intermediate?
(3) Complete the rate law for the overall reaction that is consistent with this mechanism.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 22:30
What methods could you use to solubilize calcium carbonate
Answers: 1

Chemistry, 23.06.2019 11:30
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 23.06.2019 13:50
Use the periodic table and your knowledge of isotopes to complete these statements. when polonium-210 emits an alpha particle, the child isotope has an atomic mass of 1-131 undergoes beta-minus decay. the chemical symbol for the new element is fluorine-18 undergoes beta-plus decay. the child isotope has an atomic mass of done intro donne
Answers: 1
You know the right answer?
The following mechanism has been proposed for the gas phase reaction of nitrogen monoxide with bromi...
Questions




Mathematics, 17.04.2021 03:00



Mathematics, 17.04.2021 03:00

History, 17.04.2021 03:00





Spanish, 17.04.2021 03:00

Mathematics, 17.04.2021 03:00

World Languages, 17.04.2021 03:00




Mathematics, 17.04.2021 03:00

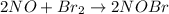
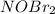 .
.![R=K[NO]^2[Br]](/tpl/images/0515/0512/945c3.png)
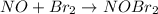 ..[1]
..[1]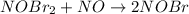 ...[2]
...[2]![R=k[NOBr_2][NO]](/tpl/images/0515/0512/7dc1f.png) ..[3]
..[3]![K_c=\frac{[NOBr_2]}{[NO][Br_2]}](/tpl/images/0515/0512/8d77b.png)
![[NOBr_2]=K_c\times [NO][Br_2]](/tpl/images/0515/0512/df1dc.png)
![[NOBr_2]](/tpl/images/0515/0512/86582.png) rate expression [3]:
rate expression [3]:![R=k\times k_c[NO][NO][NO]=K[NO]^2[Br]](/tpl/images/0515/0512/f99df.png)