
Chemistry, 19.02.2020 01:36 lolidkhelp
When solid NH4HS is placed in a closed flask at 28oC, the solid dissociates according to the equation below. NH4HS(s) ⇄ NH3(g) + H2S(g). The total pressure of the equilibrium mixture is 0.766 atm. Determine Keq at this temperature.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1


Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 23.06.2019 06:00
Nthis lab, you will do experiments to identify types of changes. using the question format you learned (shown above), write an investigative question that you can answer by doing these experiments
Answers: 3
You know the right answer?
When solid NH4HS is placed in a closed flask at 28oC, the solid dissociates according to the equatio...
Questions




Biology, 25.08.2019 03:30

Business, 25.08.2019 03:30





English, 25.08.2019 03:30

Mathematics, 25.08.2019 03:30


Mathematics, 25.08.2019 03:30







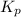 is the constant of a certain reaction at equilibrium.
is the constant of a certain reaction at equilibrium.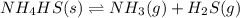
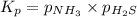
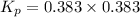
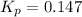
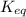 at this temperature is 0.147
at this temperature is 0.147


