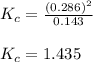Chemistry, 19.02.2020 01:51 bougiehairstudios
When 9.2 g of frozen N2O4 is added to a 0.50 L reaction vessel and the vessel is heated to 400 K and allowed to come to equilibrium, the concentration of N2O4 is determined to be 0.057 M. Given this information, what is the value of Kc for the reaction below at
400 K? N2O4(g) ⇌ 2 NO2(g)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

You know the right answer?
When 9.2 g of frozen N2O4 is added to a 0.50 L reaction vessel and the vessel is heated to 400 K and...
Questions



Mathematics, 09.05.2021 02:40


History, 09.05.2021 02:40


Mathematics, 09.05.2021 02:40


Mathematics, 09.05.2021 02:40

Mathematics, 09.05.2021 02:40


History, 09.05.2021 02:40

Spanish, 09.05.2021 02:40






Mathematics, 09.05.2021 02:40

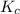 for the given reaction is 1.435
for the given reaction is 1.435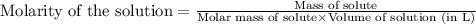
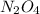 = 9.2 g
= 9.2 g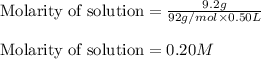
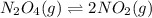
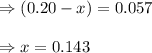
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0515/1174/271f5.png)
![[NO_2]_{eq}=2x=(2\times 0.143)=0.286M](/tpl/images/0515/1174/a94f2.png)
![[N_2O_4]_{eq}=0.057M](/tpl/images/0515/1174/d44e5.png)