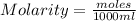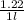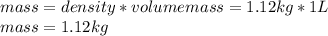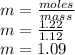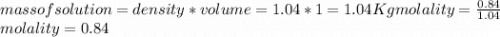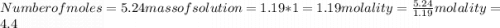Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

You know the right answer?
Calculate the molalities of the following aqueous solutions:. (a) 1.22 M sugar (C12H22O11) solution...
Questions



Mathematics, 26.11.2019 21:31

Mathematics, 26.11.2019 21:31

Physics, 26.11.2019 21:31

Mathematics, 26.11.2019 21:31





Health, 26.11.2019 21:31

English, 26.11.2019 21:31




Mathematics, 26.11.2019 21:31

Social Studies, 26.11.2019 21:31