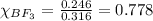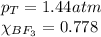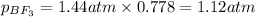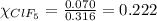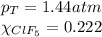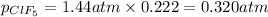Chemistry, 19.02.2020 04:02 waterborn9800
A tank at is filled with of boron trifluoride gas and of chlorine pentafluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Be sure your answers have the correct number of significant digits. a. boron trifluoride mole fraction:b. partial pressure:c. chlorine pentafluoride mole fraction:d. partial pressure:d. Total pressure in tank:

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
You know the right answer?
A tank at is filled with of boron trifluoride gas and of chlorine pentafluoride gas. You can assume...
Questions

Mathematics, 04.05.2020 23:31


English, 04.05.2020 23:31

Mathematics, 04.05.2020 23:31

Mathematics, 04.05.2020 23:31

Mathematics, 04.05.2020 23:31


Mathematics, 04.05.2020 23:31

Mathematics, 04.05.2020 23:31

Mathematics, 04.05.2020 23:31

Mathematics, 04.05.2020 23:31


Social Studies, 04.05.2020 23:31

English, 04.05.2020 23:31



Mathematics, 04.05.2020 23:31

Mathematics, 04.05.2020 23:31


Mathematics, 04.05.2020 23:31

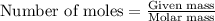 .....(1)
.....(1)
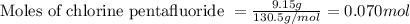
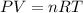
![4.19^oC=[4.19+273]K=277.19K](/tpl/images/0515/3530/8cd9a.png)
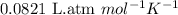
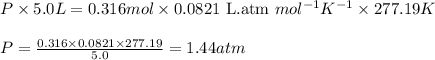
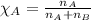 .......(2)
.......(2)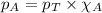 .....(3)
.....(3)