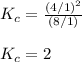Chemistry, 19.02.2020 04:25 paolacorazza
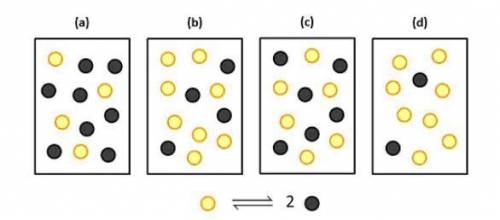
The equilibrium constant for the reaction
N2O4(g)⇌2NO2 at 2 ∘C is Kc = 2.0.
If each yellow sphere represents 1 mol of N2O4(g) and each gray sphere 1 mol of NO2 which of the following 1.0 L containers represents the equilibrium mixture at 2 ∘C?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1

Chemistry, 23.06.2019 01:30
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
The equilibrium constant for the reaction
N2O4(g)⇌2NO2 at 2 ∘C is Kc = 2.0.
If each ye...
N2O4(g)⇌2NO2 at 2 ∘C is Kc = 2.0.
If each ye...
Questions

Mathematics, 27.01.2021 02:20

Mathematics, 27.01.2021 02:20

Mathematics, 27.01.2021 02:20

Arts, 27.01.2021 02:20

Mathematics, 27.01.2021 02:20

Arts, 27.01.2021 02:20


Mathematics, 27.01.2021 02:20

Arts, 27.01.2021 02:20


Mathematics, 27.01.2021 02:20




Biology, 27.01.2021 02:20

Health, 27.01.2021 02:20

English, 27.01.2021 02:20

Mathematics, 27.01.2021 02:20


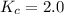 is system (b)
is system (b)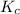
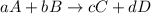
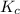 is written as:
is written as:![K_{c}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0515/3649/b6f47.png)
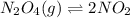
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0515/3649/271f5.png) .......(1)
.......(1)