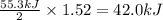A chemist measures the energy change during the following reaction: 1. This reaction is:. a. endothermic. b. exothermic. 2. Suppose 70.1 g of NO2 react. Will any heat be released or absorbed? A. Yes, absorbed. B. Yes, released. C. No. 3. If you said heat will be released or absorbed, calculate how much heat will be released or absorbed?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

You know the right answer?
A chemist measures the energy change during the following reaction: 1. This reaction is:. a. endothe...
Questions

Biology, 05.03.2021 03:50


Mathematics, 05.03.2021 03:50

Mathematics, 05.03.2021 03:50

Mathematics, 05.03.2021 03:50

Mathematics, 05.03.2021 03:50


Mathematics, 05.03.2021 03:50

History, 05.03.2021 03:50







Mathematics, 05.03.2021 03:50



Mathematics, 05.03.2021 03:50

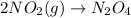
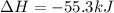 1. This reaction is:______. a. endothermic. b. exothermic.
1. This reaction is:______. a. endothermic. b. exothermic. 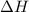 for Endothermic reaction is positive and
for Endothermic reaction is positive and 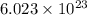 of particles.
of particles.
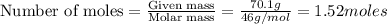
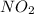 reacts, energy released = 55.3 kJ
reacts, energy released = 55.3 kJ