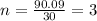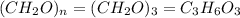Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
The empirical formula of a compound is CH2O and its molecular weight is 90.09 g/mol. Determine the m...
Questions

Social Studies, 09.11.2020 14:00

Social Studies, 09.11.2020 14:00

History, 09.11.2020 14:00

German, 09.11.2020 14:00



Mathematics, 09.11.2020 14:00




Biology, 09.11.2020 14:00




History, 09.11.2020 14:00

English, 09.11.2020 14:00

Spanish, 09.11.2020 14:00

English, 09.11.2020 14:00


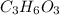
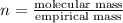
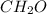 and the molar mass of compound is, 90.09 gram/mol.
and the molar mass of compound is, 90.09 gram/mol.