
Chemistry, 19.02.2020 18:42 rhineharttori
Given the balanced equation for the reaction of butane and oxygen: 2C4H10 + 13O2 > 8CO2 + 10H2O + energy How many moles of carbon dioxide are produced when 5.0 moles of butane react completely? * 5.0 mol 10. mol 20. mol 40. mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3
You know the right answer?
Given the balanced equation for the reaction of butane and oxygen: 2C4H10 + 13O2 > 8CO2 + 10H2O +...
Questions




Mathematics, 26.08.2019 09:00

English, 26.08.2019 09:00






Biology, 26.08.2019 09:00

Mathematics, 26.08.2019 09:00

Mathematics, 26.08.2019 09:00

History, 26.08.2019 09:00


Mathematics, 26.08.2019 09:00

Mathematics, 26.08.2019 09:00


Mathematics, 26.08.2019 09:00

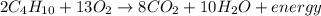
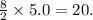 moles of carbon dioxide
moles of carbon dioxide

