Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1


Chemistry, 23.06.2019 03:00
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1
You know the right answer?
Acetylsalicylic acid (aspirin), HC9H7O4, is the most widely used pain reliever and fever reducer. Fi...
Questions



Mathematics, 22.06.2019 01:30


Mathematics, 22.06.2019 01:30

Biology, 22.06.2019 01:30

Mathematics, 22.06.2019 01:30


Mathematics, 22.06.2019 01:30

Health, 22.06.2019 01:30


Mathematics, 22.06.2019 01:30



Mathematics, 22.06.2019 01:30




Mathematics, 22.06.2019 01:30

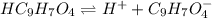
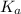 for above equation follows:
for above equation follows:![K_a=\frac{[C_9H_7O_4^-][H^+]}{[HC_9H_7O_4]}](/tpl/images/0515/8851/cb0ef.png)
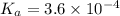
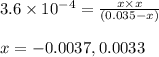
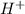 = x = 0.0033 M
= x = 0.0033 M![pH=-\log[H^+]](/tpl/images/0515/8851/cf945.png)