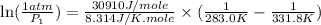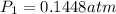Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
10. translate each of the following chemical equations into a sentence. a. 2 zns(s) + 3 o2(g) -> 2 zno(s) + 2 so2(g) b. cah2(s) + 2 h2o(l) -> ca(oh)2 (aq) + 2 h2(g)
Answers: 2

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 12:00
Marcel just purchased 1.69 grams of iron fillings in order to make living putty for his 6 year old niece. how many moles of iron are made in his sample?
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
The normal boiling point of bromine is 58.8°C, and its enthalpy of vaporization is 30.91 kJ/mol. Wha...
Questions






Chemistry, 07.10.2019 08:00

Mathematics, 07.10.2019 08:00


English, 07.10.2019 08:00



Social Studies, 07.10.2019 08:00



English, 07.10.2019 08:01


Mathematics, 07.10.2019 08:01

Mathematics, 07.10.2019 08:01


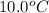 is 0.1448 atm.
is 0.1448 atm.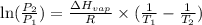
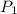 = vapor pressure of bromine at
= vapor pressure of bromine at 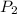 = vapor pressure of propane at normal boiling point = 1 atm
= vapor pressure of propane at normal boiling point = 1 atm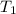 = temperature of propane =
= temperature of propane = 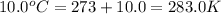
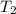 = normal boiling point of bromine =
= normal boiling point of bromine = 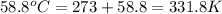
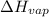 = heat of vaporization = 30.91 kJ/mole = 30910 J/mole
= heat of vaporization = 30.91 kJ/mole = 30910 J/mole