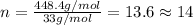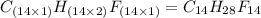Compound consists of carbon, hydrogen and fluorine. In one experiment, combustion of 2.50 g of the compound produced 3.926 g of CO2. Another sample weighing 5.00 g was found to contain 2.54 g of fluorine. The molar mass is found to be 448.4 g/mol. What are its empirical and molecular formulas? (AW(amu): C = 12.01, H = 1.008, F = 19.00)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1

Chemistry, 23.06.2019 13:40
Match these items with their examples. 1. liquid solution milk 2. solid solution aluminum foil 3. compound soda 4. colloid steel 5. element salt
Answers: 1

Chemistry, 23.06.2019 14:00
How does electronegativity changes as we move from left to right across a period
Answers: 2
You know the right answer?
Compound consists of carbon, hydrogen and fluorine. In one experiment, combustion of 2.50 g of the c...
Questions


Mathematics, 31.08.2021 03:00

Mathematics, 31.08.2021 03:00

Mathematics, 31.08.2021 03:00


History, 31.08.2021 03:00


English, 31.08.2021 03:00

Mathematics, 31.08.2021 03:00



Mathematics, 31.08.2021 03:00

Chemistry, 31.08.2021 03:00


Health, 31.08.2021 03:00

Mathematics, 31.08.2021 03:00

Mathematics, 31.08.2021 03:00



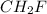 and
and 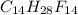 respectively
respectively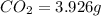
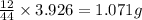 of carbon will be contained.
of carbon will be contained.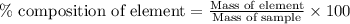 ......(1)
......(1)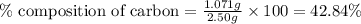
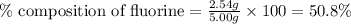
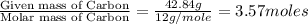
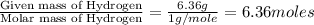
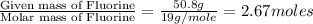
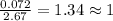
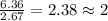
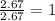
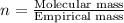
![12+(2\times 1)+19]=33g/mol](/tpl/images/0516/0009/ccdc5.png)