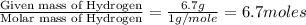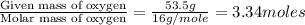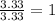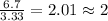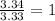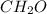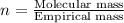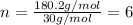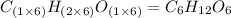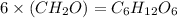Chemistry, 19.02.2020 20:44 coryowens44
Chemical analysis of an organic compound found the following composition: 40.0% C, 53.5% O, and 6.7% H. If the molar mass is 180.2 g/mol, how many empirical formula units are there in the molecular formula?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 18:30
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1
You know the right answer?
Chemical analysis of an organic compound found the following composition: 40.0% C, 53.5% O, and 6.7%...
Questions





Mathematics, 27.04.2021 16:40

Mathematics, 27.04.2021 16:40



Mathematics, 27.04.2021 16:40

Mathematics, 27.04.2021 16:40

Mathematics, 27.04.2021 16:40

Mathematics, 27.04.2021 16:40








Mathematics, 27.04.2021 16:40