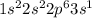Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:50
If a substance is not at its melting or boiling point, as the heat content of a sample of matter increases, its temperature increases the number of intermolecular bonds decreases the space between particles increases the particles move faster
Answers: 2

Chemistry, 22.06.2019 10:30
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 18:30
The table lists the lattice energies of some compounds.compoundlattice energy (kj/mol)lif –1,036licl –853naf –923kf –821nacl –786which statement about crystal lattice energy is best supported by the information in the table? the lattice energy increases as cations get smaller, as shown by lif and kf.the lattice energy increases as the cations get larger, as shown by lif and licl.the lattice energy decreases as cations get smaller, as shown by nacl and naf.the lattice energy decreases as the cations get smaller, as shown by naf and kf.
Answers: 3

Chemistry, 22.06.2019 23:20
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2
You know the right answer?
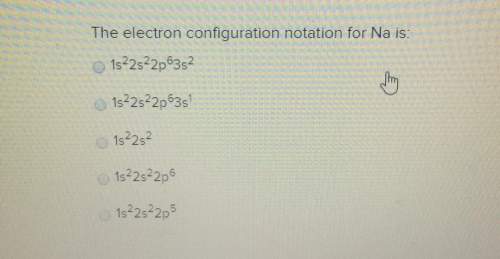
The electron configuration notation for na is: o 1s22s22p63s21s 2s 2p 3s1s22s22p61s22s22p
...
...
Questions


Mathematics, 30.11.2019 09:31


Chemistry, 30.11.2019 09:31


Physics, 30.11.2019 09:31


History, 30.11.2019 09:31

History, 30.11.2019 09:31


History, 30.11.2019 09:31

Mathematics, 30.11.2019 09:31

Computers and Technology, 30.11.2019 09:31

Arts, 30.11.2019 09:31

Mathematics, 30.11.2019 09:31





Mathematics, 30.11.2019 09:31