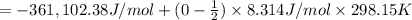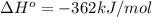A sample of K(s) of mass 2.720 g undergoes combustion in a constant volume calorimeter at 298.15 K. The calorimeter constant is 1849 J K−1, and the measured temperature rise in the inner water bath containing 1439 g of water is 1.60 K.
Part A
Calculate ΔU∘f for K2O.
Express your answer to three significant figures and include the appropriate units.
Part B
Calculate ΔH∘f for K2O.
Express your answer to three significant figures and include the appropriate units.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3

Chemistry, 23.06.2019 15:00
20 look at the clock and the data table below. based on the data and on your knowledge of potential and kinetic energy, what is the best conclusion you can make about potential and kinetic energy? the total amount of energy stays the same. the clock has the most potential energy at point b since it is moving the fastest. there is always more potential energy than kinetic energy. potential energy can never be 0, but you can have 0 kinetic energy.
Answers: 1
You know the right answer?
A sample of K(s) of mass 2.720 g undergoes combustion in a constant volume calorimeter at 298.15 K....
Questions

Biology, 09.04.2021 04:40

Chemistry, 09.04.2021 04:40

Mathematics, 09.04.2021 04:40




Biology, 09.04.2021 04:40



Mathematics, 09.04.2021 04:40

Mathematics, 09.04.2021 04:40




Mathematics, 09.04.2021 04:40


Mathematics, 09.04.2021 04:40



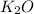 is -361 kJ/mol.
is -361 kJ/mol.![q=[q_1+q_2]](/tpl/images/0516/2162/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0516/2162/1d50b.png)
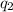 = heat absorbed by the water
= heat absorbed by the water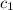 = specific heat of calorimeter =
= specific heat of calorimeter = 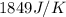
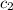 = specific heat of water =
= specific heat of water = 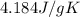
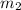 = mass of water = 1439 g
= mass of water = 1439 g = change in temperature =
= change in temperature = 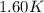
![q=[(1849 J/K \times 1.60 K)+(1439 g \times 4.184J/gK\times 1.60 K)]](/tpl/images/0516/2162/36fc3.png)
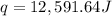
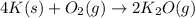
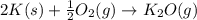
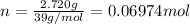
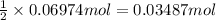 of
of 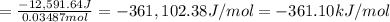
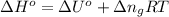
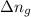 = moles of gases on RHS - moles of gasses on LHS
= moles of gases on RHS - moles of gasses on LHS