
Chemistry, 19.02.2020 22:59 Picklehead1166
Sodium metal (atomic weight 22.99 g/cm^3) adopts a body-centered cubic structure with a density of 0.97 g/cm^3. (a) Use this information and Avogrado's number (Na=6.022x10^23) to estimate the atomic radius of sodium. (b) If it didn't react so vigorously, sodium could float on water. Use the answer from part (a) to estimate the density of Na if its structure were that of a cubic close-packed metal. Would it still float on the water?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
Sodium metal (atomic weight 22.99 g/cm^3) adopts a body-centered cubic structure with a density of 0...
Questions


Social Studies, 11.10.2019 00:50

History, 11.10.2019 00:50

Spanish, 11.10.2019 00:50




Chemistry, 11.10.2019 00:50

Mathematics, 11.10.2019 00:50


Mathematics, 11.10.2019 00:50

Mathematics, 11.10.2019 00:50


Biology, 11.10.2019 00:50

Health, 11.10.2019 00:50


English, 11.10.2019 00:50



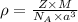
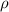 = density =
= density = 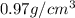
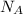 = Avogadro's number =
= Avogadro's number = 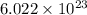
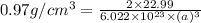
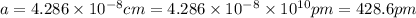
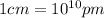
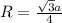
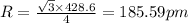
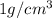
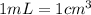 )
)

