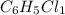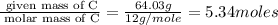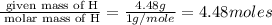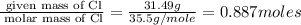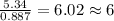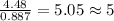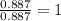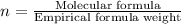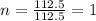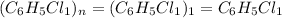Chemistry, 19.02.2020 23:18 zuleromanos
The reaction of equal molar amounts of benzene, C6H6, and chlorine, Cl2, carried out under special conditions, yields a gas and a clear liquid. Analysis of the liquid shows that it contains 64.03% carbon, 4.48% hydrogen, and 31.49% chlorine by mass and that is has a molar mass of 112.5 g/mol. The molecular formula will be determined. First, determine the number of moles of carbon in a 100 g sample.

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1


Chemistry, 23.06.2019 14:00
How much would you need to weigh out in order to have 0.2moles of magnesium atoms?
Answers: 1

Chemistry, 23.06.2019 21:20
A. explain, in terms of particles, why nacl(s) does not conduct electricity.
Answers: 1
You know the right answer?
The reaction of equal molar amounts of benzene, C6H6, and chlorine, Cl2, carried out under special c...
Questions


Mathematics, 22.03.2021 20:00

Mathematics, 22.03.2021 20:00


Mathematics, 22.03.2021 20:00


Mathematics, 22.03.2021 20:00

Biology, 22.03.2021 20:00






Mathematics, 22.03.2021 20:00




Mathematics, 22.03.2021 20:00

English, 22.03.2021 20:00